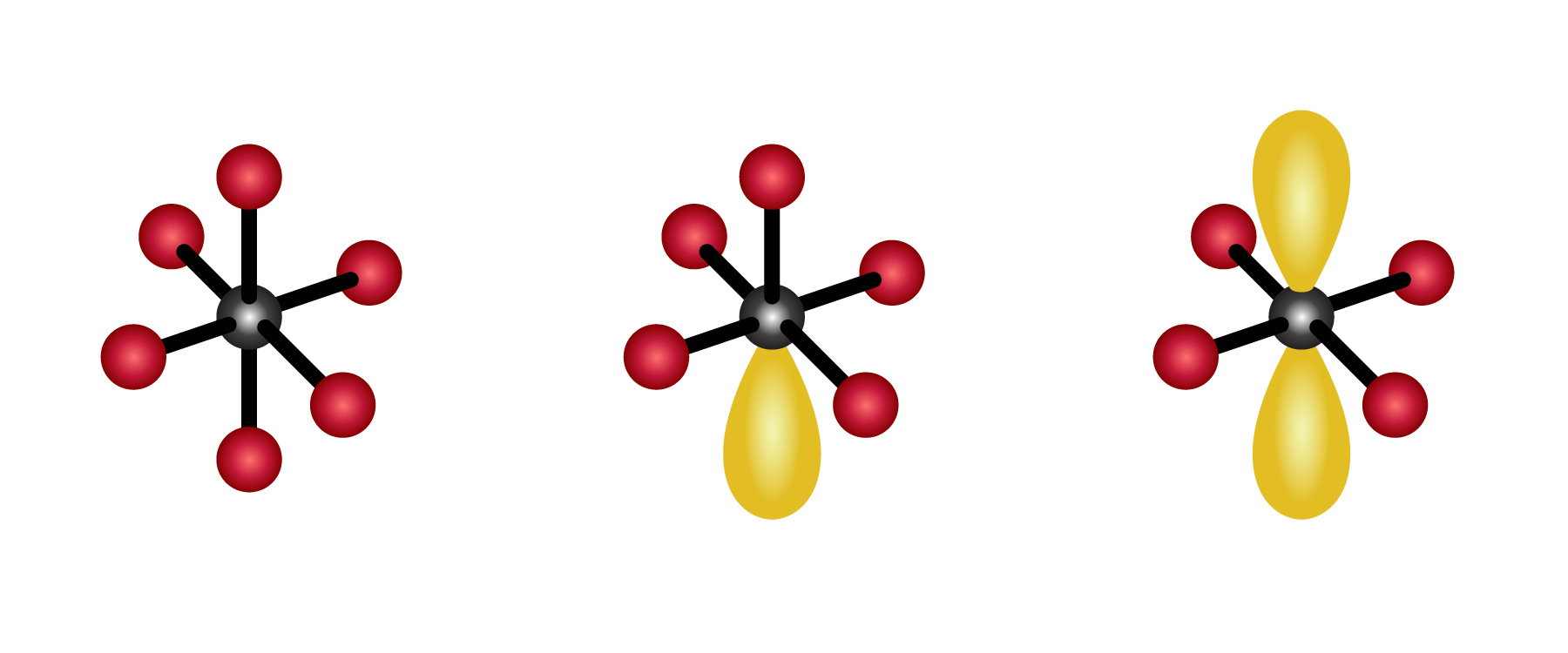
VSEPR stands for valence shell electron pair theory, and it is a very important model for predicting the geometric structure of molecules. In this webinar, we will review the basics of VSEPR, orbitals shapes, hybridization, and how VSEPR can be used to understand bonding and simple organic chemistry mechanisms that involve tetrahedral intermediates. Tune in to learn about one of the most important theories of atomic chemistry!
VSEPR