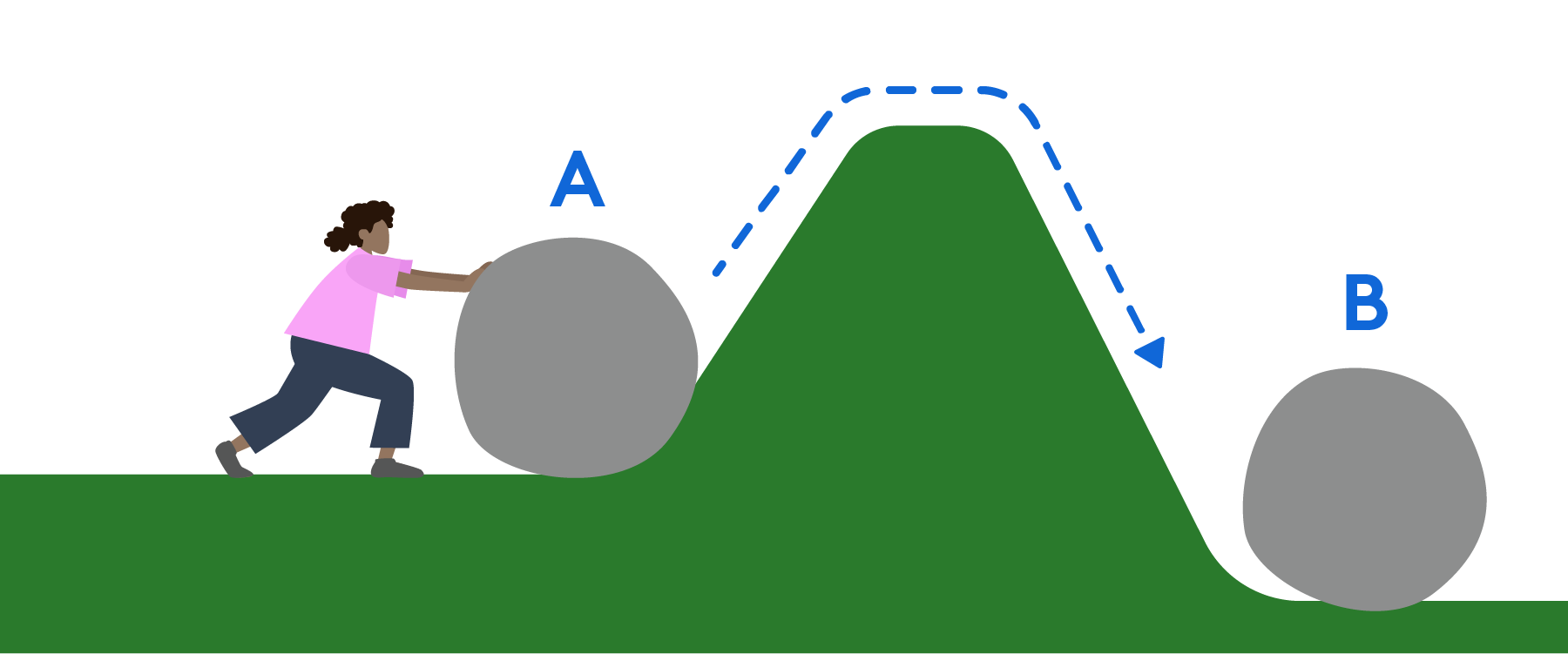
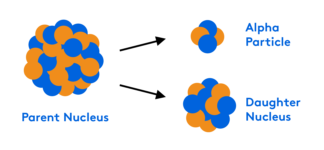
Chemical kinetics is the study of reaction rates, activation energy and catalysts. Watch this webinar to learn how to derive rate laws from experimental data or a rate determining step. We will also review the difference between transition state and intermediate, between a kinetic product and thermodynamic product. There will be some overlaps with enzyme kinetics as well as thermodynamics and equilibrium.
Kinetics